Great Thinkers: Rutherford
With this app students learn about the life and contributions to nuclear physics made by Ernest Rutherford. It discusses the early conceptual models for the structure of the atom and how Rutherfords work was a step toward the quantum model used by physicists today. Diagrams are used to illustrate key concepts.
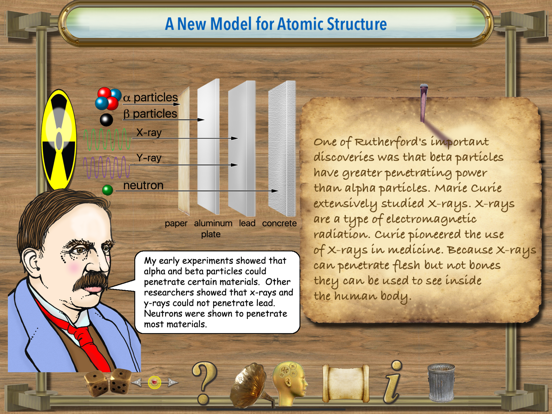
Rutherford proposed a theory to explain radioactivity. Radioactivity is the process by which certain elements, for example, uranium or radium, are in a constant state of decay. Rutherford identified the particles emanating from radioactive materials. He named the alpha and beta. The alpha particles are basically a helium atom. Beta particles are electrons. For his discovery Ernest Rutherford was awarded the Nobel prize in chemistry in 1908.
With this app students learn about the significant contributions that Ernest Rutherford made to further the development of modern physics. He was the first to reveal the nature of alpha and beta particles. He showed through his famous goid foil experiment that atoms were mostly open space.
Students will enjoy using this graphically illustrated, interactive learning tool. The timeline feature presents key events in the history of physics and tapping on timeline points brings up descriptions of each of the milestones that led to our modern understanding of atomic structure. A quiz function helps students demonstrate their comprehension of the reading material. Key concepts related to nuclear physics are shown in colorful illustrations. We hope this app will inspire students to study physics and science in general. The app shows that Ernest Rutherford certainly deserves a significant place in the history of nuclear physics.